GB 2760-2024 was released heavily, and the regulations on the use of food additives in some food categories changed.
message On March 12th, the website of the National Health Commission publicly released 47 national safety standards and 6 amendments, including Usage (GB 2760-2024). The 2014 version of GB 2760 will be officially replaced by the 2024 version of GB 2760 on February 8th, 2025. In view of the fact that the use of food additives directly affects the compliance of enterprise products, Food Partner Network has sorted out the main changes in the use of food additives in some food categories, involving the use in food, the requirements for the total amount of common use in the same food category, the restrictions on the use of sodium salts, and the restrictions on the use, etc., for industry reference.
These preservatives can’t be used in canned food.
According to the survey of canned food industry, referring to the relevant canned food product standards, that is, canned food has been pasteurized, the new version GB 2760 deleted the provisions on the use of food additives for canned food with the only function as preservative and the first function as preservative. The specific usage provisions of preservatives deleted in the new version of GB 2760 mainly include:
(1) Delete canned fruits, canned vegetables, canned nuts and seeds ε -Provisions on the use of polylysine hydrochloride;
(2) Nisin, ε -Provisions on the use of polylysine hydrochloride;
(3) Delete ε -Provisions on the use of polylysine hydrochloride, nisin, sodium diacetate (also known as sodium diacetate), sorbic acid and its potassium salt, dehydroacetic acid and its sodium salt (also known as dehydroacetic acid and its sodium salt);
(4) The regulations on the use of stable chlorine dioxide in canned aquatic products have been deleted.
The new version of GB 2760 has added the total requirements when these sweeteners are used together.
In the new GB 2760, the total amount of aspartame, acesulfame K and aspartame methyl acetylsulfamic acid used together in the same food category was increased. The specific provisions are as follows:
(1) Aspartame (also known as aspartame): If aspartame acetylsulfamic acid is allowed to be used in food categories at the same time (the maximum dosage can be converted into aspartame by multiplying it by 0.64), when mixed, the maximum dosage cannot exceed the maximum dosage of aspartame specified in the standard.
(2) Aspartame Acetylsulfamic Acid: If aspartame or acesulfame K are allowed to be used in food categories at the same time, the maximum dosage of aspartame or acesulfame K in mixed use cannot exceed the maximum dosage of aspartame or acesulfame K specified in the standard (the maximum dosage of aspartame Acetylsulfamic Acid multiplied by 0.64 can be converted into aspartame, and the maximum dosage multiplied by 0.44 can be converted into acesulfame K).
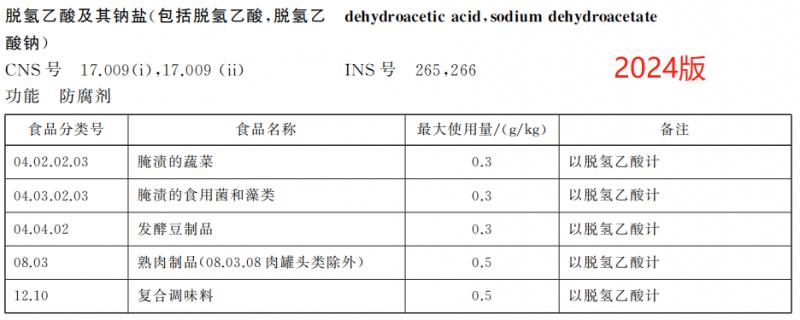
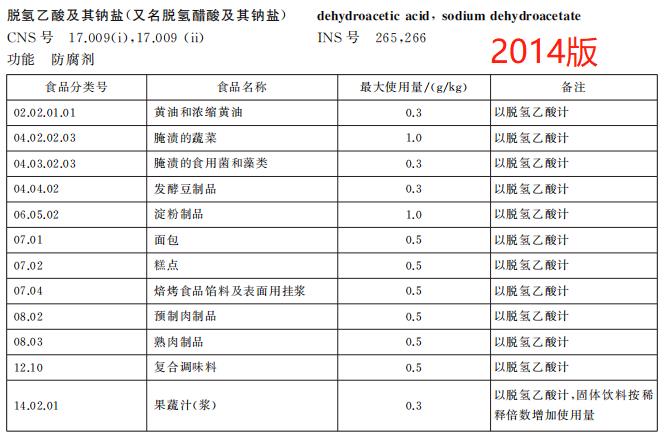
Delete the use of dehydroacetic acid and its sodium salt in 7 kinds of foods such as cakes.
According to the food safety risk assessment results of the food additive sodium dehydroacetate organized by the Food Assessment Center, combined with the investigation of the use of sodium dehydroacetate in related industries, the new version GB 2760 deleted the regulations on the use of dehydroacetic acid and its sodium salt in butter and concentrated butter, starch products, bread, cakes, baked food fillings, surface hanging pulp, prefabricated meat products, fruit and vegetable juice (pulp), and adjusted the maximum dosage of the food additive in pickled vegetables from 1.0g/kg to 0.
Limit the application range of processing aid hydrogen peroxide
Based on the latest evaluation results of safety and process necessity, combined with the actual use situation of the industry, the specific functions and application scope of hydrogen peroxide as a processing aid were clarified. The processing aid hydrogen peroxide was adjusted from the List of Processing Aids that can be used in various food processing processes and the residue is not limited to the List of Processing Aids that need to be specified in functions and application scope, and the processing aid hydrogen peroxide was specified as a desulfurizer, decolorizer and deiodifier, which can be used in starch sugar and starch processing technology, oil and fat.
Some provisions on the use of food additives have no technological necessity and are deleted.
According to the investigation of beverage industry and the principle that the use of food additives should have technological necessity, the regulations on the use of natamycin in fruit and vegetable juice were deleted.
With the improvement of production technology, taking into account the principle of technological necessity, the β -Regulations on the use of mono-and diglycerides of carotene and diacetyl tartaric acid, and the regulations on the use of azoformamide in wheat flour were deleted.
Based on the principle of process necessity, the processing aid &beta is deleted; -Provisions on the use of cyclodextrins in pasteurized milk and sterilized milk.
summarize
According to "Food safety lawThe implementation regulations stipulate that after the food safety standards are published, food producers and operators can implement them before the implementation date stipulated by the food safety standards. Although the 2024 version of GB 2760 has a transition period of nearly one year, considering that the adjustment of food formula will take time, some revisions will also involve the replacement of labels. It is recommended that enterprises involved in the above revisions review and adjust the formulas and labels in time to ensure the smooth passage of the old and new standards and ensure the compliance of production and operation.